It is increasingly evident that the world is suffering from climate change, with increasingly extreme temperatures, natural disasters becoming stronger and more frequent. These negative effects come from the pollution produced by mankind for centuries, such as, for example, the uninterrupted emission of greenhouse gases through the use of fossil fuels.
Greenhouse Gases (GHG) are a very broad group, but the most abundant is carbon dioxide (CO2) and it is used as a proportion factor for others. For example, one tonne of methane has the same effect on the atmosphere as approximately 20 to 25 tonnes of carbon dioxide, so the greenhouse effect of methane is said to be 20 to 25 tCO2e (tons of carbon dioxide equivalent) .
Concerned about climate change and its effects, European Union countries and other world powers, such as the United States, Japan, China, Canada and others, are proposing increasingly daring measures and objectives for mass decarbonization by the 2030s and with a focus on zero carbon equivalent emissions by 2050.
To achieve these goals, it is necessary to understand the energy transition, which is a paradigm shift involving the generation, consumption and reuse of energy, especially when considering that energy generation in its most varied forms is responsible for a large part of GHG emissions. worldwide.
This concept starts from the migration of polluting energy matrices, such as fossil fuels based on coal or oil, to renewable energy sources, such as solar, hydroelectric, wind and biomass. This energy transition also extends to energy efficiency, digitalization, the environment, waste management and other means, so that the common goal of reducing GHG emissions is achieved.
In this context, hydrogen (H2) produced through low or zero CO2 emission processes emerges as an alternative energy source capable of decarbonizing sectors currently known as intensive producers of GHGs, released in the burning of fossil fuels, as occurs, for example, in the cement and steel industries.
Although H2 is not an unknown substance and is already widely used in several processes, its use as a decarbonization alternative is based on the possibility of obtaining the molecule through renewable energy, as well as its use, without emitting polluting gases. , since, for example, the combustion of hydrogen releases only Water (H2O) and Oxygen (O2). However, it is still necessary to evolve in the H2 production, transport and storage processes, considering both safety aspects and technical and economic feasibility.
The hydrogen production process can be divided into three major routes: electrolytic, thermal and photolytic, being divided into seven main processes, applicable to various resources, both biomass and fossils. Processes using fossil fuels include reforming (partial oxidation, steam reforming, and partial oxidation and autothermal reforming) and the pyrolysis of hydrocarbons.
Production processes from renewable resources can be classified according to the raw material: biomass or water. Processes using biomass can be divided into two subcategories: thermochemical and biological. The former involves pyrolysis, gasification, combustion and liquefaction of biomass, while the main biological processes are biophotolysis, dark fermentation and photofermentation. The second category of renewable technologies involves the production of hydrogen from water through electrolysis, pyrolysis (thermolysis) and photolysis (photoelectrochemical decomposition) processes.
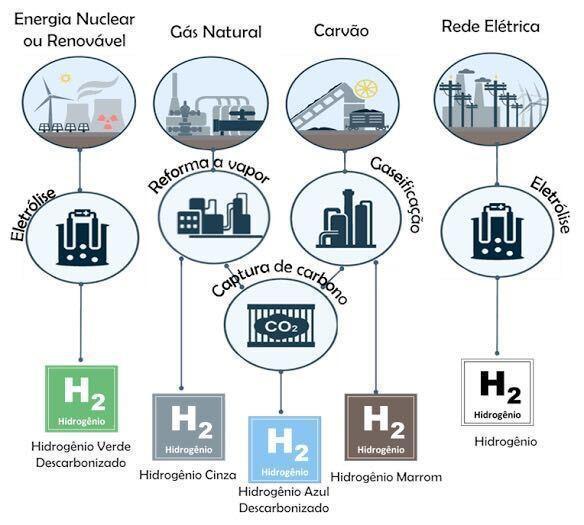
Depending on the hydrogen production route, it is categorized into colors according to its characteristics. There is still no consensus regarding this categorization, however, some classifications are being widely disseminated by institutions such as IEA, EPE, Hydrogen Council, etc., which can be summarized as follows:
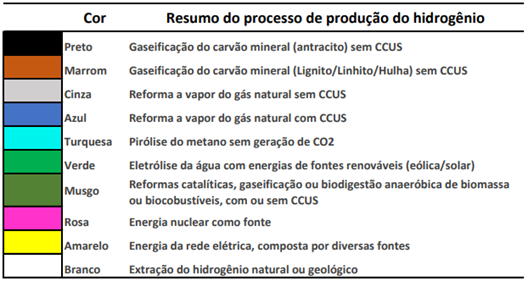
Source: Adapted from Bases for the Consolidation of the Brazilian Hydrogen Strategy (EPE, 2021)
Brown/Black Hydrogen
Produced from the gasification of mineral coal (lignite/coal – brown hydrogen, anthracite – black hydrogen) without capturing, using or sequestering the carbon dioxide resulting from the process.
Lignite has in its composition enough matter volatile, this makes it easier to convert it to a gas and to petroleum products than some coals that have a better quality. However, its high humidity and susceptibility to spontaneous combustion it causes problems in transport and storage, which makes its use more difficult, meaning that companies that use this biomass are usually located close to the mining area of this material. Another important point is that due to the high humidity and the low calorific value of lignite, emissions of carbon dioxide are usually much larger per megawatt (energy potential) generated compared to black coal and “superior” coals.
Anthracite is created by metamorphism and is associated with metamorphic rocks in the same way that bituminous coal (packed peat) is associated with sedimentary rocks. Anthracite releases high energy per pound and burns cleanly with little soot, it is also used as a medium filter, making it a more sought-after variety of coal and therefore of higher value. Mineral fossil coal was formed by the buried remains of tropical and subtropical plants, especially during the Carboniferous and Permian periods.
The gasification process occurs when carbon sources, in this case mineral coal, are exposed to air, or pure oxygen, and water vapor in a pressure vessel at very high temperatures (over 1800°C) and pressures. These high pressures and temperatures cause several reactions to occur, generating a mixture of gases called synthesis gas, usually with carbon monoxide (CO) and hydrogen (H2) in greater abundance, in addition to ash and slag in processes that use mineral sources. It is possible to apply a steam reforming process, such as the one described in the gray hydrogen below, to convert CO, which is highly harmful, to CO2.
gray hydrogen
This hydrogen in question is produced from the reforming of natural gas or coal without CCUS (carbon capture, use and sequestration). This form of production releases large amounts of carbon dioxide into the atmosphere, contributing to global warming and climate change.
The reforming of natural gas, composed mostly of methane (CH4), starts with the gas entering a reactor and receiving pre-filtration to remove the sulfur. Then, Methane, with the help of a catalyst, reacts with water vapor inside a reactor at high temperature, forming hydrogen (H2) and carbon monoxide (CO). Then, to improve the production of hydrogen in the process, another catalyst is added where carbon monoxide reacts with steam and forms carbon dioxide (CO2), thus making the final separation step where the mixed gases are separated and pure hydrogen is stored. Currently, this is the most common form of hydrogen production in the world, but there is no capture of greenhouse gases produced in the process, although a part of these gases are reused for the heating process.
Blue Hydrogen
Blue hydrogen is produced by reforming fossil fuels, just like gray, but the process is followed by capturing and storing the carbon emitted in the process. It is known as “decarbonized gas” or “low carbon gas” and is considered by some to be a clean energy source. There is controversy in this regard, as carbon capture and storage technologies are not always free from environmental problems.
This specific hydrogen is produced from fossil fuels and natural gas (mainly by the reforming method), but in this case the CCUS method (carbon capture, use and sequestration) also happens.
The CCUS process consists of storing carbon using liquids with specific catalysts that, after heating, release the gas; or there is simply a capture of carbon dioxide (CO2) directly. In both cases the CO2 is transported through pipelines to underground (“buried”) storage pockets or stored for transport. Some industries can make use of this gas, such as the fertilizer industry, the chemical industry and the methanol fuel industry.
pink hydrogen
Pink Hydrogen is produced from the electrolysis of water using nuclear energy. Water electrolysis is the chemical decomposition of water (H2O) generating the products oxygen (O2) and hydrogen (H2), by applying an electric current (energy) to the water. In this specific case, the electric current comes from the energy of nuclear reactors.
Nuclear reactors are thermoelectric plants, that is, units that generate energy by heating water, but which use the high energy resulting from reactions that take place in the nuclei of atoms as a source of energy for this heating. There are two ways to produce nuclear energy, through fission or nuclear fusion. Today only nuclear fission is commercially applicable, usually using radioactive uranium atoms. There is advanced research into making nuclear fusion commercially viable, and in 2022 it was the first time in history that it was possible to achieve a positive energy balance in this process. This type of energy can have several applications, mainly the generation of electrical energy.
Uranium, in turn, is a finite resource, although there are large reserves of this material, which means that the hydrogen from this energy source is not renewable. Despite the positive point of not producing polluting gases in the atmosphere, there are still great risks, both from the radioactive waste generated by the fission process, which is harmful to nature, and the danger that a plant in this sector brings with it (eg risk of leaks). If, in the future, nuclear fusion becomes feasible, these problems will not exist.
Turquoise Hydrogen
Produced through the pyrolysis of methane from natural gas, which is itself used as a source of energy for the thermal process. Its residue is solid carbon (coal), which is why it is considered an emission-free production.
Turquoise Hydrogen arises with the entry of methane (CH4) into a reactor heated to more than 1000ºC (celsius), which works through the use of energy from renewable sources. Later, inside the reactor, the pyrolysis (split by heat) of methane occurs, resulting in solid carbon, or coal, (C) and hydrogen (H2). In the last step, hydrogen in gas form is collected in the upper part of the reactor and the second product (coal) comes out through the lower part of the reactor in solid form, making its storage simpler. Some experts call this hydrogen “low-carbon hydrogen”.
Yellow Hydrogen
Produced by electrolysis of water with the energy to carry out the process coming from any available source.
Like Pink Hydrogen, Yellow Hydrogen is also produced from the electrolysis of water using electricity. The differential of this H2 coloring is the source of energy used in electrolysis, or in reality “the energy sources”, after all, this hydrogen is produced with a mixture of energies from sources such as renewables and fossil fuels, and it is not possible to trace the origin of the energy used. This energy comes from the networks of the National Interconnected System, which is supplied by several types of energy sources. Therefore, this hydrogen is not considered clean and renewable.
White Hydrogen
This type of hydrogen is found in its natural form, as a free gas, either in layers of the continental crust (gas pockets), at the bottom of the oceanic crust, in volcanic gases, in geysers or in hydrothermal systems. Natural/White hydrogen is present in a wide range of rock formations and geological regions. It arises from a variety of natural sources such as diagenetic origin (iron oxidation) in sedimentary basins, radiolysis (natural electrolysis) or bacterial activity, also from hyperalkaline sources containing dihydrogen (2H2) emissions, among other forms.
White hydrogen is usually exploited by ground drilling methods. But, currently, there are not many strategies and plans to exploit this type of hydrogen, as the other “colors” are much more advantageous and practical to be acquired and used.
Hydrogen Moss
The gasification process, in the case of biomass, takes place in 4 stages: the first is drying, in which the biomass loses its retained water; in the second stage, pyrolysis is used to start the decomposition of the biomass and prepare it for the next stage; in the third stage, the combustion of this product takes place; and in the fourth stage, the reduction of the material takes place, where carbon and hydrocarbons from the fuels used partially react with oxygen and generate carbon monoxide (CO), which is highly harmful, and hydrogen gas (H2). This process takes place at very high temperatures, from 900°C.
This gas mixture, produced in the fourth step, can later be converted to nothing more than hydrogen and carbon dioxide (CO2) by adding steam and reacting over a catalyst in a water gas displacement reactor, facilitating the use of gas technology. CCUS, if it is included in the process.
Green Hydrogen
Also produced from the electrolysis of water using electricity, as well as Pink and Yellow. In this case, the electricity used in the process must necessarily come from renewable sources that do not emit GHGs, mainly hydro, wind and solar. It is the cleanest hydrogen production alternative, as it is free of greenhouse gas (GHG) emissions, and as such has received strong support from governments and companies around the world.
Therefore, it is believed that green hydrogen is one of the “fuels of the future”, since it could be used in several applications that today are difficult to decarbonize, such as in the steel, cement, fertilizers, glass, among others, as well as as an alternative fuel for vehicles, aircraft and ships.
There is still a long way to go, as green hydrogen currently represents a small percentage of the global hydrogen market and is not yet economically viable. However, similar to what happened with the introduction of renewable energy sources in the energy mix, the tendency is for the cost of producing green hydrogen to reduce as it gains economies of scale and becomes a safer and more mature process from the point of view of from a technological and financial point of view.
Comments are closed.

